water hardness test formula|calculate water hardness calculator : factory The test strips method is a simpler and quicker way to measure water hardness. The test strips are coated with a reactive material that changes color when exposed to calcium and magnesium ions. The user can then compare the color of the strip to a chart to determine the level of hardness. This method is less accurate than titration but is .
8 de jan. de 2024 · ️ o tarot de hoje na cartomancia e o dom cigano com henrique cigano hoje☎️📱+55(41) 995 37 43 66 whats para agendar consulta falar com luciana 🔥💥seja memb.
{plog:ftitle_list}
Sinopse: Tinker Bell, também conhecida como Sininho, é uma fada do verão que habita o Vale das Fadas. Curiosa e arteira, ela decide quebrar uma importante regra e vai parar no Bosque do Inv.
Temporary Hard Water. Temporary hard water is hard water that consists primarily of calcium (Ca 2 +) and bicarbonate (HCO 3-) ions.Heating causes the bicarbonate ion in temporary hard water to decompose into .Water Hardness Calculations. . When performing the hardness test through titration, hardness as mg CaCO 3 /L can be determined with the following formula (only when the titration factor is 1.00 of EDTA, otherwise add the .
Table 01: Categories of water based on the hardness. EDTA titrimetric method of determining the total hardness of water. Ca 2+ and Mg 2+ ions in the water sample are titrated with Ethylenediaminetetraacetic acid .The formula for calculating water hardness involves multiplying the concentration of calcium ions by a factor of 2.5 and the magnesium ions by a factor of 4.1. These values are then summed up to provide the total water hardness, expressed in mg/L as calcium carbonate (CaCO₃). . Temporary and permanent hardness would require additional .Water hardness is the measurement of the number of ions that have lost two electrons (divalent cations) dissolved in the tested water and is, therefore, related to total dissolved solids. The more divalent cations dissolved in the water, the "harder" the water. Generally, the most common divalent cations are calcium and magnesium.The test strips method is a simpler and quicker way to measure water hardness. The test strips are coated with a reactive material that changes color when exposed to calcium and magnesium ions. The user can then compare the color of the strip to a chart to determine the level of hardness. This method is less accurate than titration but is .
Water hardness test meters detect the number of solids dissolved in your water. Find the “On” button near the meter’s display and press it down for a few seconds. After 2-3 seconds, the display will light up and read “0.0” so you can start using the meter. [7]General Chemistry II Lab #4 - Determination of the Hardness of Water 1 One of the factors that establishes the quality of a water supply is its degree of hardness. The hardness of water is defined in terms of its content of calcium and magnesium ions. . neutralization; its structural formula is below. N N HO O OH O O HO O OH3.2 Conducting the Test. Positioning the Sample: Secure the material sample in the testing machine.; Selecting the Indenter: Choose the appropriate ball diameter based on the material hardness.; Applying the Load: Gradually apply the specified load using the machine.The load should be maintained for a predetermined dwell time, usually between 10 to 15 seconds.
total permanent hardness = calcium hardness + magnesium hardness The calcium and magnesium hardness is the concentration of calcium and magnesium ions expressed as equivalent of calcium carbonate. The molar mass of CaCO 3 , Ca 2+ and Mg 2+ are respectively 100,1 g/mol, 40,1 g/mol and 24,3 g/mol. If you have measured the total water hardness using EBT as the endpoint and the concentration of the Ca 2+ using HB as the endpoint, how could you determine the concentration of Mg 2+? Since the concentration of the Al 3+ and the iron species are usually very low to negligible, the Mg 2+ can be determined by taking the difference in results . The Vickers Hardness test (ISO 6507) is used to characterize hardness of various solid materials (metals, ceramics, etc.). A diamond pyramid is pressed against the solid with a certain normal load and the hardness is calculated based on the imprint left on the surface.
water hardness dh to ppm
Hardness of water is because of the presence of salts of calcium and magnesium. Know the different Types of Hardness in water and the process to Remove Temporary and Permanent Hardness. . Test Your Knowledge On Hardness Of Water Types And Removal! Q 5. Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz .Laboratory Experiment 5: Hardness Objective: Measure (1) Total hardness and (2) Calcium hardness using dye indicators Background: Hard Water: Hard waters are generally considered to be those waters that require considerable amounts of soap to produce foam and that also produce scale in water pipes, heaters, boilers and
📏 Method 3: Hard Water Test Strips. Hard water testing strips offer a quick and easy way to test for hard water at home. A DIY water hardness test works by changing color to indicate the minerals present in the water. You .
Determining the total hardness in water using complexometric titration with EDTA tutorial suitable for chemistry students. . EDTA prepared in this way will have the formula Na 2 H 2 C 10 H 12 O 8 N 2.2H 2 O Molar mass of this .
Using a Test Kit: You can purchase a water hardness test kit at most hardware stores or online. These kits typically include test strips that change color based on your water’s hardness level. Professional Testing: .
Understanding the hardness of your water is essential for maintaining appliances, ensuring effective cleaning, and making informed decisions about water treatment. Here are three methods for testing water hardness at home: Using an At-Home Test Kit. At-home water hardness test kits are convenient and accessible tools that provide quick results .2340 A. Introduction 1. Terminology Originally, water hardness was understood to be a measure of the capacity of water to precipitate soap. Soap is precipitated chiefly by the calcium and magnesium ions present. Other polyvalent cations also may precipitate soap, but they often are in complex forms, frequently with organic constituents, and their role in water hardness may be .Originally, the hardness of water was understood to be a measure of the capacity of water for precipitating soap. Soap is precipitated chiefly by the calcium and magnesium ions commonly present in water, but may also be precipitated by ions of other polyvalent metals, such as aluminium, iron, manganese, strontium and zinc, and by hydrogen ions. .
Water hardness can be measured using a titration with ethylenediaminetetraacetic acid (EDTA). At a pH of 10, calcium and magnesium ions form colorless, water soluble, complexes with EDTA. . After titration you can determine hardness through the following formula: Example: If 22 mL of EDTA were used to titrate a sample to the end point of a .December 18, 2013 hard water, Quality test determination of hardness of water by edta method, edta and calcium, edta magnesium, . Determination of hardness of water by EDTA method is one of the three main methods for determination of hardness of water. With calcium and magnesium ion, this reagent can forms a stable complex. .Estimation of Hardness of Water by EDTA Method 3 In the pH range 8-10, the blue form of the indicator HD2– gives a wine red complex with Mg2+: Mg+2 + HD 2– MgD– + H+ (Blue) (Wine red) Now if EDTA (H2Y 2–) is added to such a solution Mg2+ preferentially complexes with EDTA (since the metal EDTA complex is more stable than the metal-indicator complex) and liberates .
A bathtub faucet with built-up calcification from hard water in Southern Arizona.. Hard water is water that has a high mineral content (in contrast with "soft water"). Hard water is formed when water percolates through deposits of limestone, chalk or gypsum, [1] which are largely made up of calcium and magnesium carbonates, bicarbonates and sulfates.. Drinking hard water may . As water hardness is usually reported in terms of mg/L of calcium carbonate (even if water contains both calcium and magnesium), we will use for calculations slightly strange reaction equation: CaCO 3 + EDTA 4-→ CaEDTA 2-+ CO 3 2-That allows direct calculation of calcium carbonate mass for known amount of titrant used.Calcium hardness is best monitored with a drop-count titration, as test strips can only measure total hardness. In addition, whereas test strips for total hardness have just four or five color blocks to cover a tremendously wide range—from 0 to 1,000 parts per million (ppm) with color blocks for 0, 100, 250, 500, 1,000, for instance—a drop .
Hardness (as mg CaCO 3 /L) = 2.497•[Ca, mg/L] + 4.118•[Mg, mg/L] Eq. 9. Florida Surface Water Quality Criteria. FDEP is charged with protecting the quality of state-owned surface water bodies with respect to their designated uses. While no water hardness criteria are specified, metals criteria are based on water hardness.
water hardness converter calculator
Gas Permeability Test System mfg
Gas Permeability Test System trade
Gas Permeability Test System traders
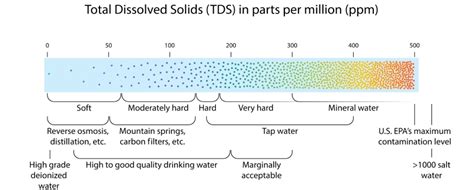
water hardness chart
Resultado da O site da BRLWON oferece jogos com uma experiência de aventura de .
water hardness test formula|calculate water hardness calculator